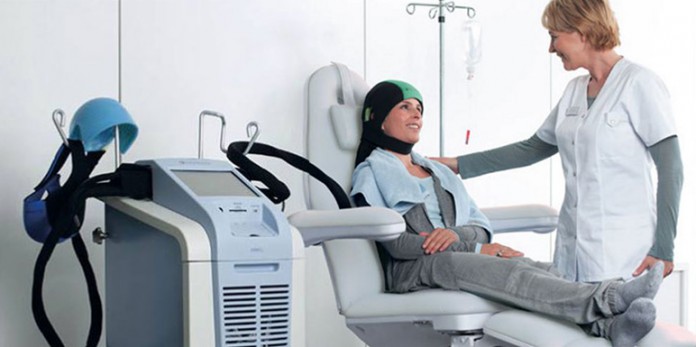
DigniCap® scalp cooling system is the first and only scalp cooling device approved by the Food and Drug Administration of U.S (FDA) to reduce chemotherapy related hair loss in women with breast cancer. The DigniCap® system has proved that during the FDA clinical trials in the U.S., seven out of 10 patients with early-stage breast cancer kept at least 50% of their hair.
However, this DigniCap® system is now only available at the following ten cancer treatment centers across the United States:
- Charleston Hematology Oncology Associates, Charleston, South Carolina
- Chesapeake Oncology and Hematology Associates, Glen Burnie, Maryland
- Florida Cancer Specialists & Research Institute, Sarasota, Florida
- Mount Sinai Comprehensive Cancer Center, Miami Beach, Florida
- Orange County Blood and Cancer Care, Fountain Valley, California
- The Angeles Clinic and Research Institute, an affiliate of Cedars-Sinai, Los Angeles, California
- Toledo Clinic Cancer Center, Toledo, Ohio
- UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, California
- UT Health Science Center San Antonio’s Cancer Therapy & Research Center,
San Antonio, Texas - Wake Forest Baptist Medical Center, Winston Salem, North Carolina

The DigniCap® scalp cooling system features a patented tight-fitting silicone cooling cap that is placed directly on the head, and an outer neoprene cap that insulates and secures the silicone cap. The cooling cap is connected to a cooling and control unit with touch screen prompts. A liquid coolant circulates throughout the silicone cap, delivering consistent and controlled cooling to all areas of the scalp. The cap is fitted to the head, and the temperature of the scalp is lowered, resulting in vasoconstriction with reduced delivery of chemotherapy to the scalp, as well as reduced cellular uptake of drugs due to decreased intra follicular metabolic rate. These factors together reduce the risk of chemotherapy-induced hair loss.
To learn more about the DigniCap® scalp cooling system, visit www.DigniCap.com.